Poietis, a pioneer in bioprinting, and the Assistance Publique – Hôpitaux de Marseille (“AP-HM”) announced today the installation of the first bioprinting platform compatible with the regulatory requirements surrounding the manufacture of implantable tissues in patients, within the Advanced Therapy Medicinal Product (ATMP) manufacturing area of the Hôpital de la Conception. As one of the first results of the collaboration initiated in early 2020 between the Cell Culture and Therapy Laboratory (“LCTC”) of the AP-HM and the Poietis company, this installation is a major milestone in the roadmap to start the first clinical trial of a 3D bioprinted human tissue, which will be a skin substitute bioprinted from patients’ cells.
Recent research in the field of tissue engineering and bioprinting has led to extremely promising results, paving the way for new therapeutic strategies for tissue repair and/or substitution as well as for personalised medicine. However, the current manufacturing methods of biological tissues are not satisfactory enough to be adopted by the medical community. Manufacturing processes need to be standardised and treatments made affordable. Although various bioprinting technology platforms have been developed in recent years to meet these challenges, none of them has proved compatible with the Good Manufacturing Practices regulations associated with the manufacture of advanced therapy medicinal products (ATMPs). Therefore, no bioprinted biological tissue has yet been implanted in a patient.
The NGB bioprinting platform, which was developed by Poietis and recently installed in the LCTC of the AP-HM, meets these challenges thanks to numerous technological advances. The integration of laser-assisted bioprinting and 3D extrusion biomaterial printing technologies in a completely aseptic environment, the robotization of the platform and the automation of the processes allow the production of functional tissues with a high level of reproducibility and flexibility. Thanks to this new platform, the printing speed has also been increased by a 1,000, thus allowing to print 40cm² of skin substitutes in a few hours.
“Bioprinting is entering a new era. The development of the first bioprinting platform that complies with the Good Manufacturing Practices (GMP) of ATMPS is a breakthrough innovation in the field of regenerative medicine as it removes one of the last barriers before grafting bioprinted tissues into patients”, says Fabien Guillemot, President and Founder of Poietis.
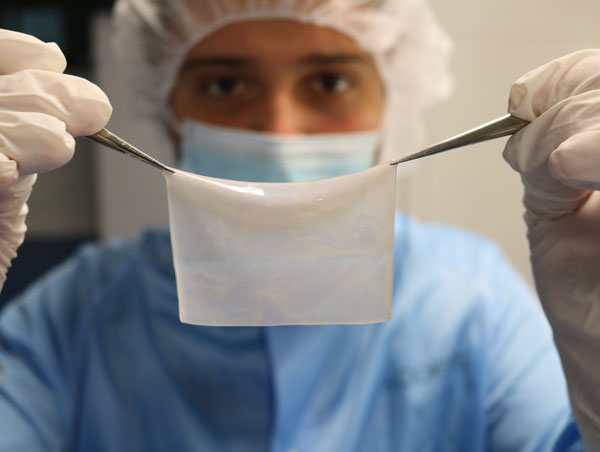
Poieskin bioprinted skin substitute
Dr Maxime Abellan-Lopez, a plastic surgeon at AP-HM, adds: “Bioprinting allows us to obtain a large quantity of skin thanks to a simple sample taken very easily from the patient. This is the therapeutic innovation we have been waiting for. It offers us the capacity to reconstruct the skin of patients requiring a graft, such as after a burn or serious trauma, with minimal adverse effects “.
Thanks to the latest recommendations from the French Agency for the Safety of Health Products, LCTC will finalize the validation of the Poieskin skin substitute manufacturing process before being able to start a clinical trial next year, which will be carried out with the support of the Plastic and Reconstructive Surgery Department and the Interregional Center for severe burns at AP-HM. “The local and national ecosystem is very favorable and really contributes to the progress of this ambitious project” underlines Dr Julie Véran, Production Manager at the LCTC.
Dr. Jérémy Magalon, a pharmacist-biologist at LCTC, says: “We are close to the possibility of grafting human tissue manufactured via a bioprinter, which would be a world first. This is the result of a multidisciplinary collaboration that has succeeded in successively overcoming the technological and regulatory barriers intrinsic to this cutting-edge innovation. But this is only step one. The goal is to bioprint more complex tissues at the point of care, as closely to the patient as possible. In the future, we can envision all major hospitals being equipped with bioprinters. This reminds me of the success of surgical robotics: a disruptive innovation that has become essential in daily practice”.
“This installation is a very important milestone that will allow us to launch the commercialization of NGB-C bioprinters to Cell Therapy and Translational Research Centers” concludes Bruno Brisson, co-founder and Business Development Director of Poietis, “It will also help us accelerate the development of our portfolio of implantable bioprinted tissues for wound healing, in the osteoarticular field and for treating neurodegenerative diseases”.

